IBM Clinical Development Review
 OUR SCORE 98%
OUR SCORE 98%
- What is IBM Clinical Development
- Product Quality Score
- Main Features
- List of Benefits
- Technical Specifications
- Available Integrations
- Customer Support
- Pricing Plans
- Other Popular Software Reviews
What is IBM Clinical Development ?
IBM Clinical Development is an innovative electronic data capture (EDC) solution built to streamline clinical research. It is a robust cloud-based software that enables users in the life science sector to gain visibility into clinical trial data. The platform provides a powerful site, patient, and trial data management tools. IBM Clinical Development is fully customizable, and users can tailor the solution to fit their unique requirements, a specific facet of a clinical trial, or other needs regardless of the stage or size of the clinical trial. The electronic data capture engine is built at the heart of IBM Clinical Development, allowing users to create and unify clinical trial data. It also enables users to access study data with ease from any web-based device.Product Quality Score
IBM Clinical Development features
Main features of IBM Clinical Development are:
- Endpoint adjudication
- Reporting and analytics
- Data integration
- Randomization
- Trial Supply Management (RTSM)
- Medical Coding
- Quality and compliance
- Patient engagement (ePRO)
IBM Clinical Development Benefits
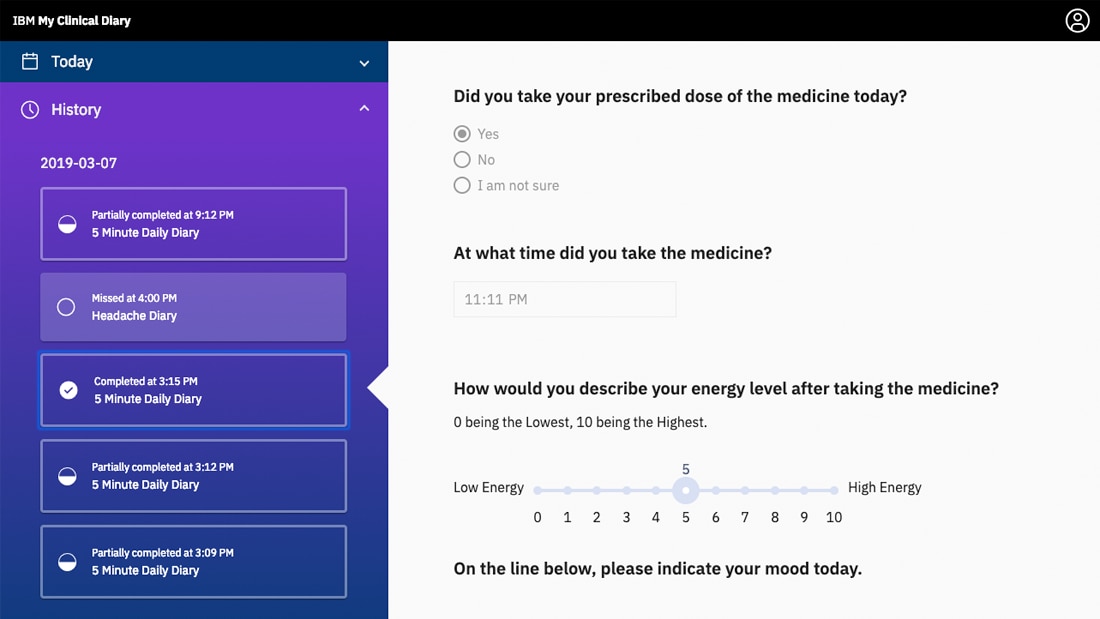
The main benefits of IBM Clinical Development are its ease of use, effectively manage clinical trials, and comprehensive data management. Here are more details:
Easy to Use
IBM Clinical Development is a SaaS-based solution that requires no prior programming skills or special infrastructure to implement and use. The solution is built with all users in mind, and it emphasizes ease of use and intuitiveness. The platform provides a password-protected interface that makes it a breeze to navigate the basic functions and access all study data.
Effectively Manage Clinical Trials
Also, IBM Clinical Development provides robust tools that make it easy to create and implement trial eCRF. The solution enables users to create clinical trial frameworks and manage all stages with unprecedented convenience, confidence, and control. Besides, the IBM service and support teams, guide users on how to create and implement effective trials, execute research, collect and document results, and manage data.
Comprehensive Data Management
Better still, IBM Clinical Development is a powerful data management solution for life science teams. The platform provides robust data management tools that let users collect and record clinical trial data. Also, it is easy for users to incorporate data from external sources into their studies. Its data migration module helps users move existing data to the IBM Clinical Development platform.
Technical Specifications
Devices Supported
- Web-based
- iOS
- Android
- Desktop
Customer types
- Small business
- Medium business
- Enterprise
Support Types
- Phone
- Online
IBM Clinical Development Integrations
The following IBM Clinical Development integrations are currently offered by the vendor:
No available information.
Video
Customer Support
Pricing Plans
IBM Clinical Development pricing is available in the following plans:





